Abstract
Introduction
Philadelphia positive (Ph+) acute lymphoblastic leukemia (ALL) presents with an aggressive clinical course and carries a high risk for relapse. The addition of tyrosine kinase inhibitors (TKI) has revolutionized the induction and maintenance treatment for this disease and has increased the patients achieving and maintaining complete remission. Allogeneic hematopoietic stem cell transplantation (HSCT) is the standard of care at this time for patients who achieve first complete remission (CR1); however, its impact on outcomes is debated. This systematic review and meta-analysis aimed to compare the outcomes of TKI maintenance therapy with or without HSCT in Ph+ ALL patients in CR1.
Methods
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, a comprehensive literature search was conducted on PubMed, Cochrane, and Clinical trials.gov using MeSH terms and keywords for " Philadelphia chromosome ", "hematopoietic stem cell transplantation" and " protein kinase inhibitors " from date of inception to May 01, 2021. A total of 1691 records were discovered using database searching. All search results were imported into the Endnote X9.0 reference manager, and duplicates were removed. The primary and secondary screening was performed, and after excluding irrelevant and review articles, a total of 6 (3 retrospectives, 3 phase II randomized controlled trials) original articles were included reporting separate outcomes of TKI maintenance therapy (TKI cohort) and HSCT followed by TKI maintenance therapy (HSCT-TKI cohort) for Ph+ ALL in CR1. Quality evaluation was done using the NIH quality assessment tool. The inter-study variance was calculated using the Der Simonian-Laird Estimator. Proportions along with a 95% Confidence Interval (CI) were extracted to compute pooled analysis using the 'meta' package by Schwarzer et al. in the RevMan (version 5.4-1).
RESULTS:
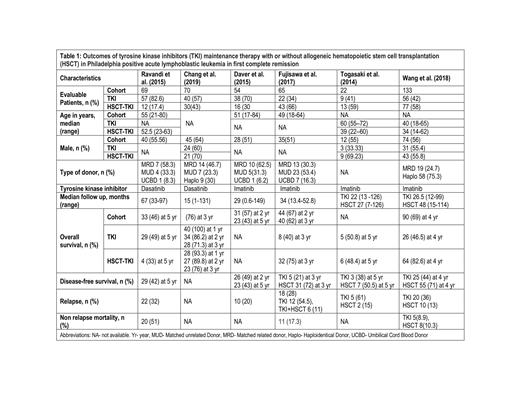
A total of 413 patients from 6 studies were included for this systematic review and meta-analysis. Out of these, 222 (53.8%) had TKI maintenance after consolidation therapy and 191 (46.2%) patients had HSCT followed by TKI maintenance in CR1. The median age of the whole cohort was 49.3 (17-84) years, while the median age for HSCT-TKI patients was 47 (14-84) years. The proportion of males was 26.2% (n=90) in TKI group versus 37.5% (n=137) in the HSCT-TKI group, as reported by 5 studies. The median follow-up was 28 (0.6-149) months. Imatinib mesylate (first-generation TKI) was used in 55.6% (n=125) patients and Dasatinib (second-generation TKI) was given to 44.4% (n=100) patients. (Table 1) Overall survival (OS) was reported from 100% and 93.3% at 1 year to 49% and 33% at 5 years for TKI and HSCT-TKI cohorts, respectively. At median follow-up time of 4 (3-5) years, a trend towards poor OS was seen with TKI group as compared to HSCT-TKI group (OR 0.46, 95% CI 0.17-1.23, I 2=70%); however, the association was not statistically significant. Disease-free survival (DFS) was reported from 49.3% and 71.3% at 4 years to 38.1% and 50.5% at 5 years for TKI and HSCT-TKI cohorts, respectively. At a median follow-up time of 4 (3-5) years, the pooled analysis showed poor DFS for TKI as compared to HSCT-TKI cohort (OR 0.30, 95% CI 0.17-0.53, I 2=0%). Four studies (n=292) reported separate outcomes for relapse, it was 59.1% (87/147) for TKI versus 14.5% (n=21/145) for HSCT-TKI patients, and odds of developing relapse were higher among patients receiving TKI as compared to HSCT-TKI (OR=8.08, 95% CI=3.81-17.14, I 2=0%).
Conclusion:
Hematopoietic stem cell transplant in Philadelphia positive acute lymphoblastic leukemia in first complete remission followed by tyrosine kinase inhibitor maintenance therapy confers disease-free and overall survival benefit compared to tyrosine kinase inhibitors maintenance alone. Our findings confirm HSCT as a standard of care in Ph+ ALL in CR1 followed by TKI maintenance and the need for a prospective randomized clinical trial to validate these findings.
Lin: AbbVie, Aptevo Therapeutics, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Novartis, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding. Abhyankar: Incyte/Therakos: Consultancy, Research Funding, Speakers Bureau. McGuirk: Astelllas Pharma: Research Funding; Fresenius Biotech: Research Funding; EcoR1 Capital: Consultancy; Novartis: Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Pluristem Therapeutics: Research Funding; Bellicum Pharmaceuticals: Research Funding; Novartis: Research Funding; Gamida Cell: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal